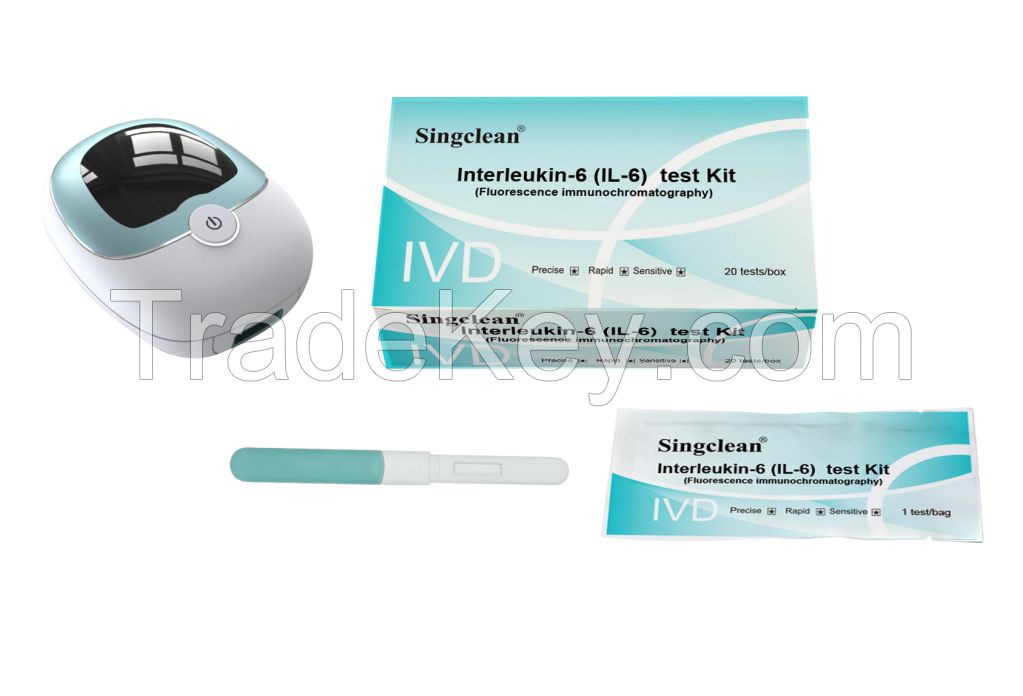
Interleukin-6 (IL-6) Test Kit (Fluorescence Immunochromatography)
- Location: Hangzhou, China
- FOB Price: 8 ~ 15.5 / Piece ( Negotiable )
- Minimum Order Quantity: 1000 Piece
- Packaging Detail: box packing with bag packing inside
- Delivery Time: 7-15days for air delivery Day
- Supplying Ability: 10000 Piece / Day
- Payment Type: Other, Western Union, T/T
Product Description
Interleukin-6 (IL-6) Test Kit
(Fluorescence Immunochromatography)
1.Introduction
Interleukin-6 is mainly produced by Th2 cells and secreted by T lymphocytes, B lymphocytes, fibroblasts, endothelial cells, keratinocytes, hepatocytes and bone marrow cells. Its relative molecular weight is 26kDa. It is a glycoprotein composed of 212 amino acid residues. Interleukin-6 is the first biomarker to appear after inflammatory reaction. In different inflammatory diseases, the content of interleukin-6 is significantly different. The increased level of interleukin-6 caused by bacterial infection is significantly higher than that caused by non-bacterial infection, and the level of interleukin-6 is directly proportional to the severity of inflammation and infection. Interleukin-6 is also a sensitive early warning index of sepsis, and has a good correlation with the severity and prognosis of sepsis. As an inflammatory factor, the level of interleukin-6 is also closely related to cardiovascular and cerebrovascular diseases such as atherosclerosis and acute myocardial infarction (AMI).
2. Indications for Use
The kit is used for quantitative determination of interleukin-6 (IL-6) in human serum, plasma or whole blood samples in vitro.
3. Who should have the Interleukin-6 (IL-6) Test Kit Test?
Patients suffering from inflammatory conditions;Patients with diabetes, stroke or with cardiovascular disease and COVID-19-infected patients. Interleukin-6 (IL-6) may be used to help evaluate a person who has a condition associated with inflammation, such as lupus or rheumatoid arthritis, or with infection, such as sepsis. It may also be used in the evaluation of diabetes, stroke, or cardiovascular disease.
Besides, Interleukin-6 (IL-6) Test Kit can help determine the risk of developing severe coagulation-related consequences such as disseminated intravascular coagulation (DIC) and acute respiratory distress syndrome (ARDS) in severe cases of COVID-19 infection.
Combined with the highly specific PCT joint inspection, it can early warning of sepsis, reflect the severity of infection, evaluate the treatment effect, and help the clinic to quickly judge the patient's situation
4.Product information
| Product Name | Interleukin-6 (IL-6) Test Kit (Fluorescence Immunochromatography) |
| Method | Fluorescence immunochromatography |
| Specimen | Whole blood, Plasma, Serum |
| Specification | 10 tests/box, 20 tests/box, 25 tests/box, 30 tests/box, 50 tests/box, 100 tests/box. |
| Components | Each pouch contains a test card and a desiccant; the test card is composed of a shell and a test strip, the test strip is composed of sample pad, fluorescent pad (fixed with fluorescent labeled IL-6 monoclonal antibody 1), nitrocellulose membrane (coated with IL-6 monoclonal antibody 2 and goat anti-mouse IgG), filter paper and PVC plastic plate |
| Test time | 15 mins |
Fluorescence immunoassay analyzer
It should be purchased separately and can be used for different Test Items, like CK-MB, IL6, PCT.
5. Features
● Qualitative, Rapid and High Sensitive
● Easy to administer and read results
● For Early detection of inflammation
6.Applicable instrument
Fluorescence immunoanalyzer
7. Storage conditions and validity period
The test kit should be stored in a sealed aluminum foil bag at 4℃~30℃, and the validity period is 18 months. After the foil bag is opened, the validity period is 1 hour.
8. Sample requirements
1. Human serum, plasma or whole blood samples; other body fluids and samples may not give accurate results.
2. Venous blood or fingertip blood should be collected under sterile conditions. It is recommended to use human serum or plasma for testing.
3. Anticoagulation with EDTA, sodium citrate or heparin is recommended for plasma and whole blood samples.
4. After the clinical blood samples are collected, the test must be completed within 4 hours at room temperature; serum and plasma can be stored at 2~8°C for 3 days and stored below -20°C for 5 months. Whole blood samples should not be frozen and stored at 2~8°C for 3 days. Avoid heat inactivating samples, and hemolyzed samples should be discarded.
5. Samples must be returned to room temperature before testing. Cryopreserved samples need to be completely thawed, rewarmed, and evenly mixed before use. Do not freeze and thaw repeatedly.
9. Test process
Please read the instructions of the test kit and the manual of the fluorescence immunoanalyzer carefully before use.
a) Bring the test kit and sample to be tested to room temperature.
b) Make sure the ID card matches the batch number of the kit, and insert the ID card into the card reading area of the instrument to read the information.
c) Open the inner package of the test card, take out the test card; draw 75μL of serum plasma sample, drop vertically to the test card sampling place, and start timing; draw 75μL whole blood, vertically drop it to the test card sampling place, and immediately add 1 drop whole blood diluent at the sampling place and start timing.
d) After adding the sample, click "Start Test" on the screen of the fluorescence immunoanalyzer, and the test card will react at room temperature for 15 minutes; insert the test card into the test card slot of the fluorescence immunoanalyzer, and the instrument will automatically test the test card; The test results can be seen on the display screen of the immunoassay analyzer. Click "Print" on the screen to print the results.
10. Positive judgment value or reference interval
Normal reference value:< 7pg/mL。
Due to differences in geography, race, gender and age, it is recommended that each laboratory establish its own positive judgment value or reference interval.
11. Interpretation of test results](For reference only, not used as clinical diagnostic criteria, test results need to be combined with other clinical and laboratory data for clinical diagnosis)
| Content of IL-6(pg/mL) | Clinical application suggestions |
| < 7 | Normal value |
| 7-150 | Indicates mild inflammation or mild infection. |
| 150-250 | Suggests general bacterial infection or systemic inflammatory reaction. |
| >250 | Suggests may be sepsis. |
12. Limitations
a) This test kit is only for the test of human serum, plasma or whole blood samples. Abnormal hematocrit samples have an impact on the results of the whole blood test. The test results of serum, plasma and whole blood are not significant difference when the hematocrit is between 21% and 48%.
b) The test results need to be combined with other clinical and laboratory data, and if the IL-6 test results do not match the clinical assessment, further testing is required.
c) False positive results can be caused by: cross-reaction of antibody-like components in the blood; some non-specific components in blood have similar antigenic determinants to capture fluorescent-labeled antibodies, such as anisotropic antibodies and rheumatoid factors. In view of the above reasons, the test results should be combined with the patient's medical history and other laboratory results performed.
d) Hemoglobin, triglycerides and bilirubin in the blood sample can interfere with the test results, where the maximum allowable concentrations are 5g/L, 10g/L and 0.2g/L, respectively.
e) False positive results can be caused by: some unknown components shield the antigenic determinants from binding to the antibody; the unstable IL-6 antigen gradually degrades with time and temperature and cannot be recognized by the antibody. Valid test results depend on a good reagent and sample storage environment.
f) Other factors, including technical reasons, operational errors and other sample factors, may also cause errors in test results.
13. Certificate
CE ISO13485
Contact Information
- Company: Hangzhou Singclean Medical Products Co., Ltd.
- Address: Hangzhou, Zhejiang, China
- Telephone: 86-18806512950
- Mobile: 86-18806512950
Company Profile
- Hangzhou Singclean Medical Products Co., Ltd.
- [ China ]
-

-
Hangzhou Singclean Medical Products Co., Ltd. was established in 2002, and currently has 18 years of expertise in the industry of absorbable biomaterial with nearly 400 employees. The production base covers an area of 28,000 square meters, with 8 GMP purification workshops of 2,000 square meters, a logistics center of 4,000 square meters, and a R&D center and a quality inspection center of 1,00...
Basic Information
- Business Type: Manufacturing
- Company Products / Services: hyalurnic acid,covid-19 test kit,absorbable hemostatic gauze,absorbable hemostatic particles,dermal filler,hyaluronic acid skin care products
- Year Established: 2002
- Number of Employees: 101-500
- Website: http://www.singclean.net/