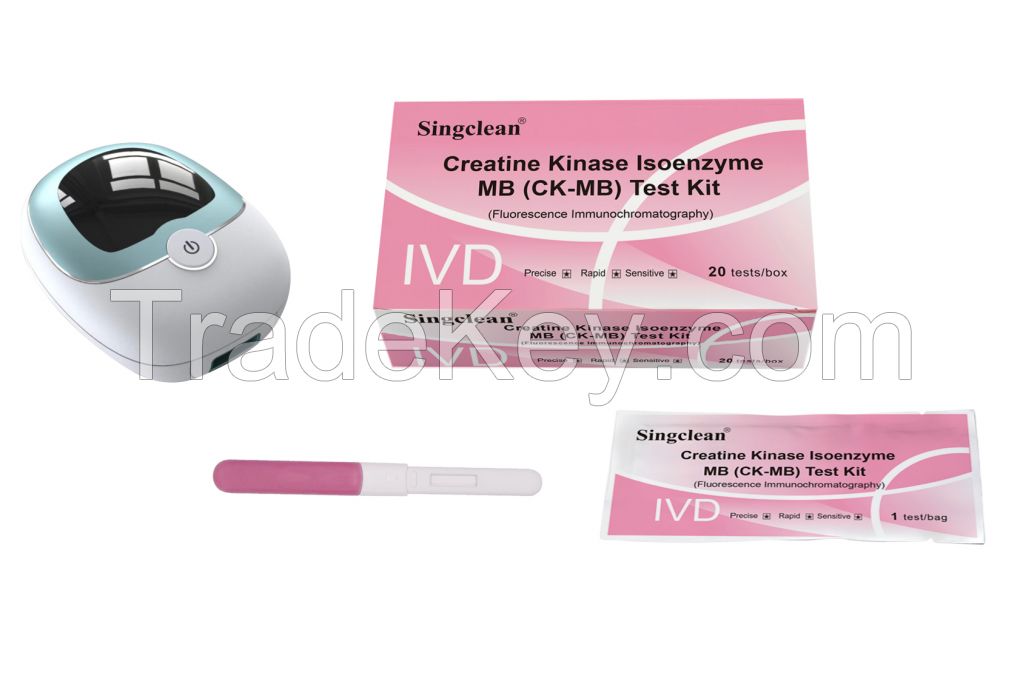
Creatine Kinase Isoenzyme MB (CK-MB) Test Kit With Fluorescence Immunochromatography)
- Location: China
- FOB Price: 8 ~ 15.5 / Piece
- Minimum Order Quantity: 1000 Piece
- Packaging Detail: Carton Packing with bag packing inside
- Delivery Time: 6-15days Day
- Supplying Ability: 10000 Piece / Day
- Payment Type: Other, Western Union, T/T
Product Description
Creatine Kinase Isoenzyme MB (CK-MB) Test Kit (Fluorescence Immunochromatography)
1. Introduction
Creatine Kinase Isoenzyme MB (CK-MB) is one form of Creatine Kinase (CK). The normal range in blood is less than 5ng/ml, which is not detected in normal blood, or only trace CK-MB is present. Only after myocardial injury, the myocardial cell ruptured and creatine kinase released from the myocardial cell and absorbed into the blood, can the creatine kinase be detected in blood sample, which indicates the presence of myocardial injury. Singclean® Creatine Kinase Isoenzyme MB (CK-MB) Test Kit (Fluorescence Immunochromatography) is used for quantitative determination of Creatine Kinase Isoenzyme MB (CK-MB) range in human serum, plasma or whole blood samples in vitro as the mark of myocardial infarction.
2. Indications for Use
This kit is used for the quantitative determination of Creatine Kinase Isoenzyme MB (CK-MB) in human serum, plasma or whole blood samples in vitro.
There are four isozyme forms of Creatine Kinase (CK): muscle type (MM), brain type (BB), mixed type (MB) and mitochondrial type (MiMi), among which MB type is mainly present in myocardial cells. In myocardial infarction, creatine kinase increases within 6 hours of onset, peaks at 24 hours, and returns to normal within 3~4 days. Among them, Creatine Kinase Isoenzyme MB has high diagnostic specificity, so it has become one of the markers of myocardial infarction.
3. Who should have a MB (CK-MB) Test?
Unnormal range of MB (CK-MB) in blood is the sign of myocardial infarction.
If you have symptoms like angina, arrhythmia, heart failure and compressive pain or tightness in precordium that lasts more than 30 minutes and it is suggested to have a MB (CK-MB) testing.
By testing the MB (CK-MB) in blood, we can know whether a persons MB (CK-MB) range is more than 5ng/ml, which means the likelihood of myocardial infarction. For those people whose MB (CK-MB) range is more than 5ng/ml, it shows they have the risk of myocardial infarction, and they should go for a timely medical treatment.
4. Singclean Advantages:
Accurate:the fluorescence immunochromatography method based test kit has higher accuracy.
Easier to read:based on fluorescence immunochromatography, easier to read test result than colloidal gold immunochromatography.
Easy for storage:it can be stored at room temperature (4℃~30℃).
Longer validity period: 18 months of validity period.
5. Product performance index
a) Accuracy: The recovery rate is between 85% and 115%.
b) Linear range: within the linear range of 2.5ng/mL~80ng/mL, the linear correlation coefficient r≥0.9900;
c) Blank limit: not higher than 2.5ng/mL;
d) Precision:
In-batch precision: The coefficient of variation (CV) is not more than 15%;
Precision between batches: The relative range between batches is not more than 15%.
6.Specification
Creatine Kinase Isoenzyme MB (CK-MB) Test Kit (Fluorescence Immunochromatography)
Format: 10 tests/box, 20 tests/box, 25 tests/box, 30 tests/box, 50 tests/box, 100 tests/box.
7. Main components
| No. | Name | Component |
| 1 | Creatine Kinase Isoenzyme MB (CK-MB) Test Kit (Fluorescence Immunochromatography) | Each bag contains a test card and a desiccant; the test card consists of shell and a test strip, and the test strip consists of sample pad, fluorescent pad (fixed with fluorescently labeled CK-MB monoclonal antibody 1), Nitrocellulose membrane (coated with CK-MB monoclonal antibody 2 and goat anti-mouse IgG), filter paper and PVC plastic sheet |
| 2 | Whole Blood Diluent | 1 bottle of diluent for 10 tests/box, 20 tests/box, 25 tests/box and 30 tests/box; 2 bottles of diluent for 50 tests/box; 4 bottles of diluent for 100 test/box; 3.0mL/bottle |
| 3 | ID card | 1 pc/box |
Fluorescence immunoassay analyzer
It should be purchased separately and can be used for different Test Items, like CK-MB, IL6, PCT.
8. Specimen collection
1. Human serum, plasma or whole blood samples; other body fluids and samples may not give accurate results.
2. Venous blood or fingertip blood should be collected under sterile conditions. It is recommended to use human serum or plasma for testing.
3. Anticoagulation with EDTA, sodium citrate or heparin is recommended for plasma and whole blood samples.
4. After the clinical blood samples are collected, the test must be completed within 4 hours at room temperature; serum and plasma can be stored at 2~8°C for 3 days and stored below -20°C for 5 months. Whole blood samples should not be frozen and stored at 2~8°C for 3 days. Avoid heat inactivating samples, and hemolyzed samples should be discarded.
5. Samples must be returned to room temperature before testing. Refrigerated samples need to be completely thawed, rewarmed, and evenly mixed before use. Do not freeze and thaw repeatedly.
9. Test Procedure
Please read the instructions of the test kit and the manual of the fluorescence immunoanalyzer carefully before use.
a) Bring the test kit and sample to be tested to room temperature.
b) Make sure the ID card matches the batch number of the kit, and insert the ID card into the card reading area of the instrument to read the information.
c) Open the inner package of the test kit, take out the test card; draw 70μL of serum plasma sample, drop vertically to the sampling place of the test card, and start timing; draw 70μL whole blood, drop vertically to the sampling place of the test card, and immediately add 1 drop of whole blood diluent at the sampling place and start timing.
d) After adding the sample, click "Start Test" on the screen of the fluorescence immunoanalyzer, and the test card will react at room temperature for 10 minutes; insert the test card into the test card slot of the fluorescence immunoanalyzer, and the instrument will automatically test the test card; The test results can be seen on the display screen of the fluorescence immunoanalyzer. Click "Print" on the screen to print the results.
10. Interpretation of Results:
(For reference only, not used as clinical diagnostic criteria, test results need to be combined with other clinical and laboratory data for clinical diagnosis)
| Content of CK-MB (ng/mL) | Clinical application advice |
| 0-5 | Normal level |
| >5 | It is suggested that the patient is at risk of myocardial infarction. Note: CK-MB exceeds the normal level within 4~8 hours after myocardial infarction; peaks within 12~24 hours; and returns to normal level in about 3 days. |
11. Limitations
a) This test kit is only for the test of human serum, plasma or whole blood samples. Abnormal hematocrit samples have an impact on the results of the whole blood test. The test results of serum, plasma and whole blood are not significant difference when the hematocrit is between 21% and 48%.
b) The test results need to be combined with other clinical and laboratory data, and if the CK-MB test results do not match the clinical assessment, further testing is required.
c) False positive results may be caused by: cross-reaction of antibody-like components in the blood; some nonspecific components in blood have similar antigenic determinants to capture fluorescent-labeled antibodies.
d) Hemoglobin, triglycerides and bilirubin in the blood sample can interfere with the test results, with the maximum allowable concentrations being 5g/L, 10g/L and 0.2g/L, respectively.
e) False negative results may be caused by: some unknown components shield the antigenic determinants from binding to the antibody; the unstable CK-MB antigen gradually degrades with time and temperature and cannot be recognized by the antibody. Effective test results depend on good reagent and sample storage environment.
f) Other factors, including technical reasons, operational errors and other sample factors, may also cause errors in test results.
12. Certification
CE ISO13485
Contact Information
- Company: Hangzhou Singclean Medical Products Co., Ltd.
- Address: Hangzhou, Zhejiang, China
- Telephone: 86-18806512950
- Mobile: 86-18806512950
Company Profile
- Hangzhou Singclean Medical Products Co., Ltd.
- [ China ]
-

-
Hangzhou Singclean Medical Products Co., Ltd. was established in 2002, and currently has 18 years of expertise in the industry of absorbable biomaterial with nearly 400 employees. The production base covers an area of 28,000 square meters, with 8 GMP purification workshops of 2,000 square meters, a logistics center of 4,000 square meters, and a R&D center and a quality inspection center of 1,00...
Basic Information
- Business Type: Manufacturing
- Company Products / Services: hyalurnic acid,covid-19 test kit,absorbable hemostatic gauze,absorbable hemostatic particles,dermal filler,hyaluronic acid skin care products
- Year Established: 2002
- Number of Employees: 101-500
- Website: http://www.singclean.net/