Aqua Seal Korea Dermal Filler
- Location: South Korea
- FOB Price: ( Negotiable )
- Minimum Order Quantity: 10 Box
- Packaging Detail: 200box with a large box
- Delivery Time: ICN FOB 5days Day
- Supplying Ability: 60000 Box / Month
- Payment Type: T/T, L/C, Western Union, PayPal
Product Description
Product Description
Aqua Seal SR, BR, HR 1ml
Aqua Seal is made by Linbio Co., Ltd on its expert technology. We specialize in the aesthetic medical area and have focused on developing, manufacturing, and exporting hyaluronic acid filler.
Linbio Co., Ltd main products include injectable dermal, which also provide top-class OEM and ODM services as customer demand. Linbio Co., Ltd has been certified by ISO13485. Our factory has up to GMP production standard, the annual production capability is 60ton of Sodium hyaluronate, 3million pieces of dermal fillers. We currently supply over 75 countries and regions such as North America, South America, Europe, Middle East, South east Asia.
Product Feature
1.FILLSYS is manufactured safely and completely through a manufacturing process of 4 to 5 weeks.
2 The concentration of Aphrodite is 24 mg/ml, which is suitable for use as an HA Dermal filler.
3. It has a safe and long maintenance period, ensuring the best quality.
Product Specification/Models
* Dermal filler INFORMATION*
Ingredient : Hyaluronic acid (HA) cross-linked with BDDE
Feature : Colorless and transparent gel
Content : 10ml prefilled syringe
Storage : 1~29 Temperature
Expiry date : *24 months due to manufacture
Applicable areas : Forehead Lines (Worry Lines), Glabellar Lines (Frown Lines), Nasolabial Folds, Periorbital Lines, Oral Commissures, Acene Scar
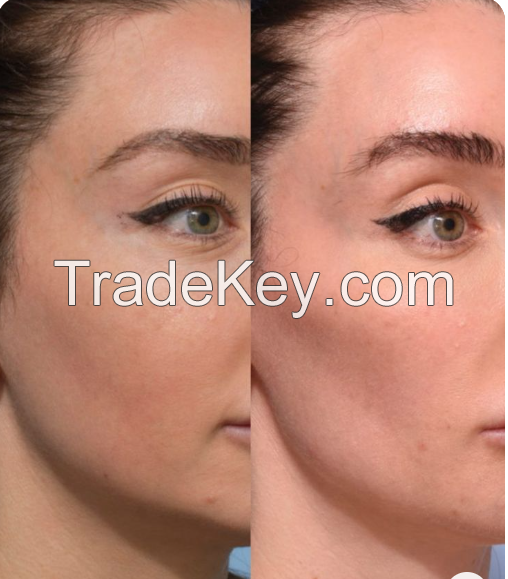
Before and after

Contact Information
- Company: Linbio Co., Ltd
- Address: South Korea
- Telephone: 82-1068789685
- Mobile: 821068789685
Company Profile
- Linbio Co., Ltd
- [ South Korea ]
-

-
We are specialized in the aesthetic medical area and has been focus on development ,manufacturing and exporting of hyaluronic acid filler.
Linbio CO.,LTD main products are include injectable dermalwe also provide top class OEM, ODM services as customer demand.
Linbio Bio CO.,LTD has been certified by ISO13485. Our factory has up to GMP production standard, the annual production capability...
Basic Information
- Business Type: Manufacturing
- Company Products / Services: dermal filler, ha filler, asteria
- Year Established: 2024
- Number of Employees: 26-50
- Website: www.linbiokorea.com








